
Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy
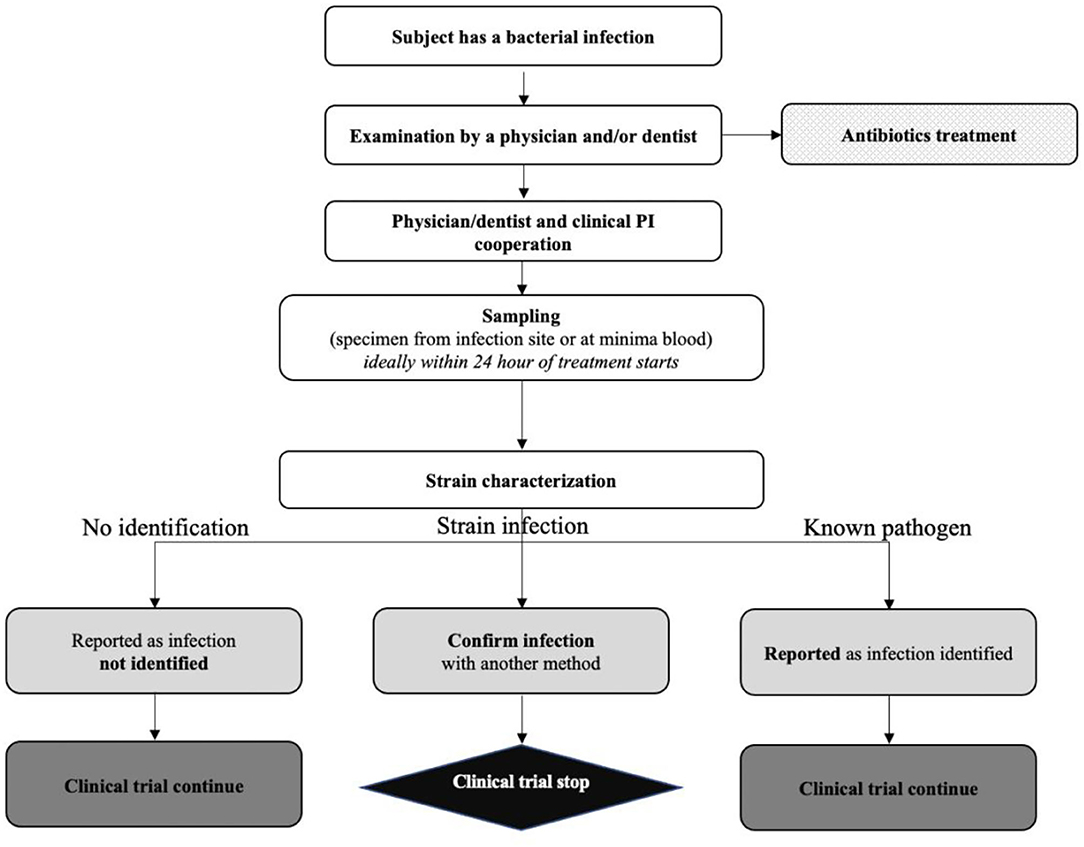
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA
EMA consultation: Guidelines on the requirements for quality documentation concerning medicinal products in clinical trials - Acron




















