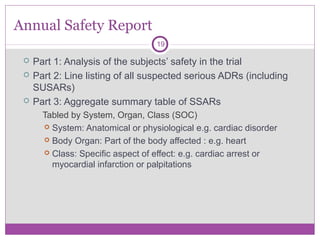
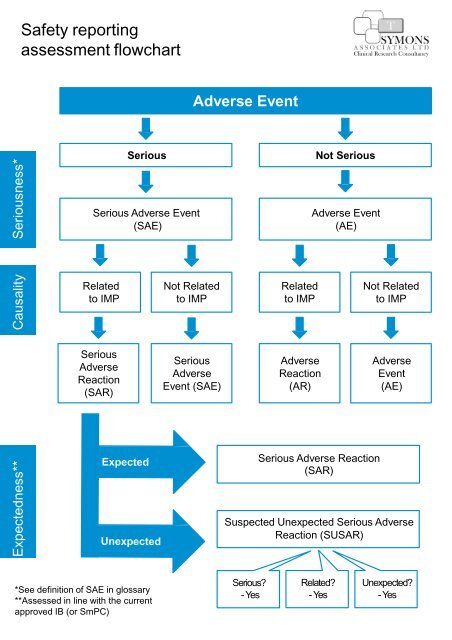
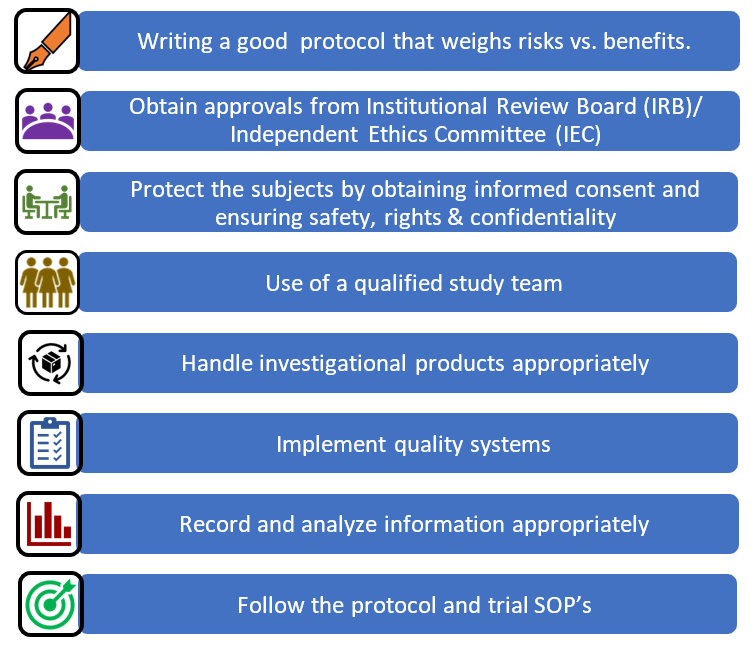
European Medicines Agency on LinkedIn: Clinical Trials Information System: training and support - European…
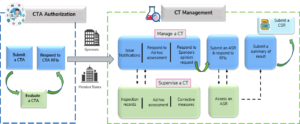
Adapting to the Evolving European Clinical Trial Regulatory Scenario: An Overview of the Current State of the European Clinical Trials Regulation and Clinical Trials Information System - ACRP