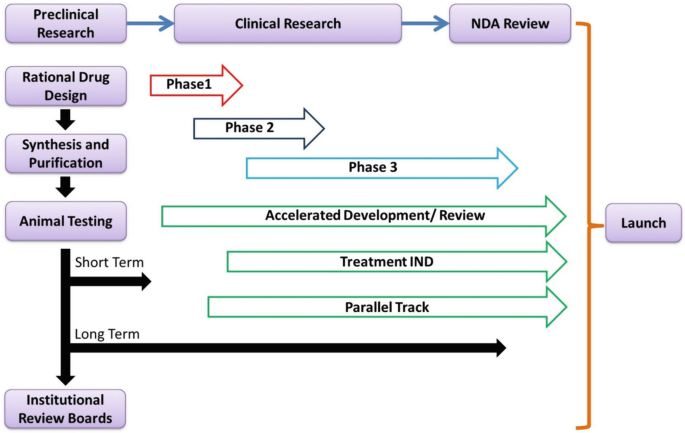
Rise of Clinical Trials Industry in India: An Analysis – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on CyberLeninka open science hub.
Welcome and Overview - International Workshop on ethical and GCP aspects of the acceptance of clinical trials submitted in Marketing Authorisation Applications to EMA.
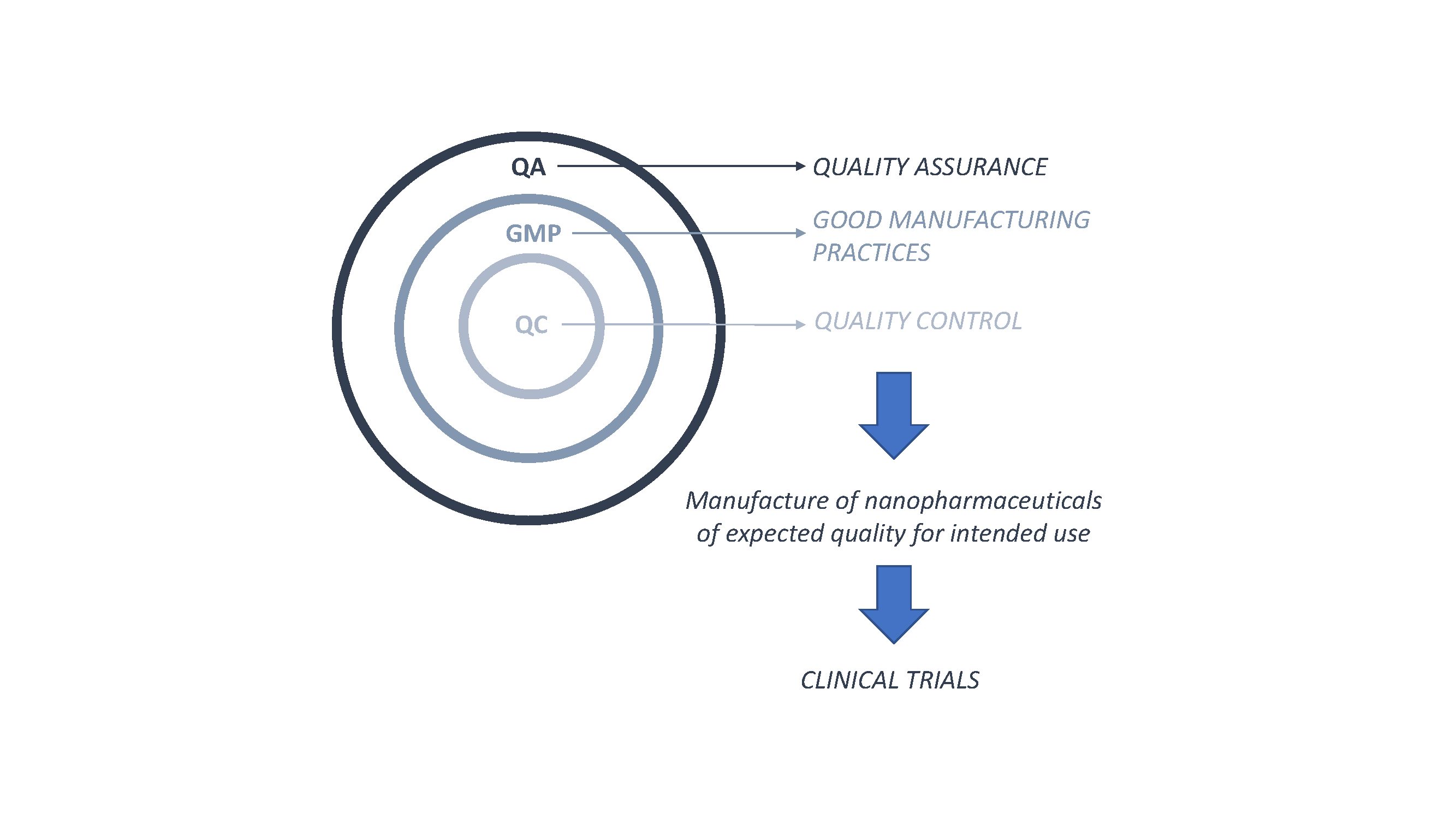
Pharmaceutics | Free Full-Text | Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU | HTML
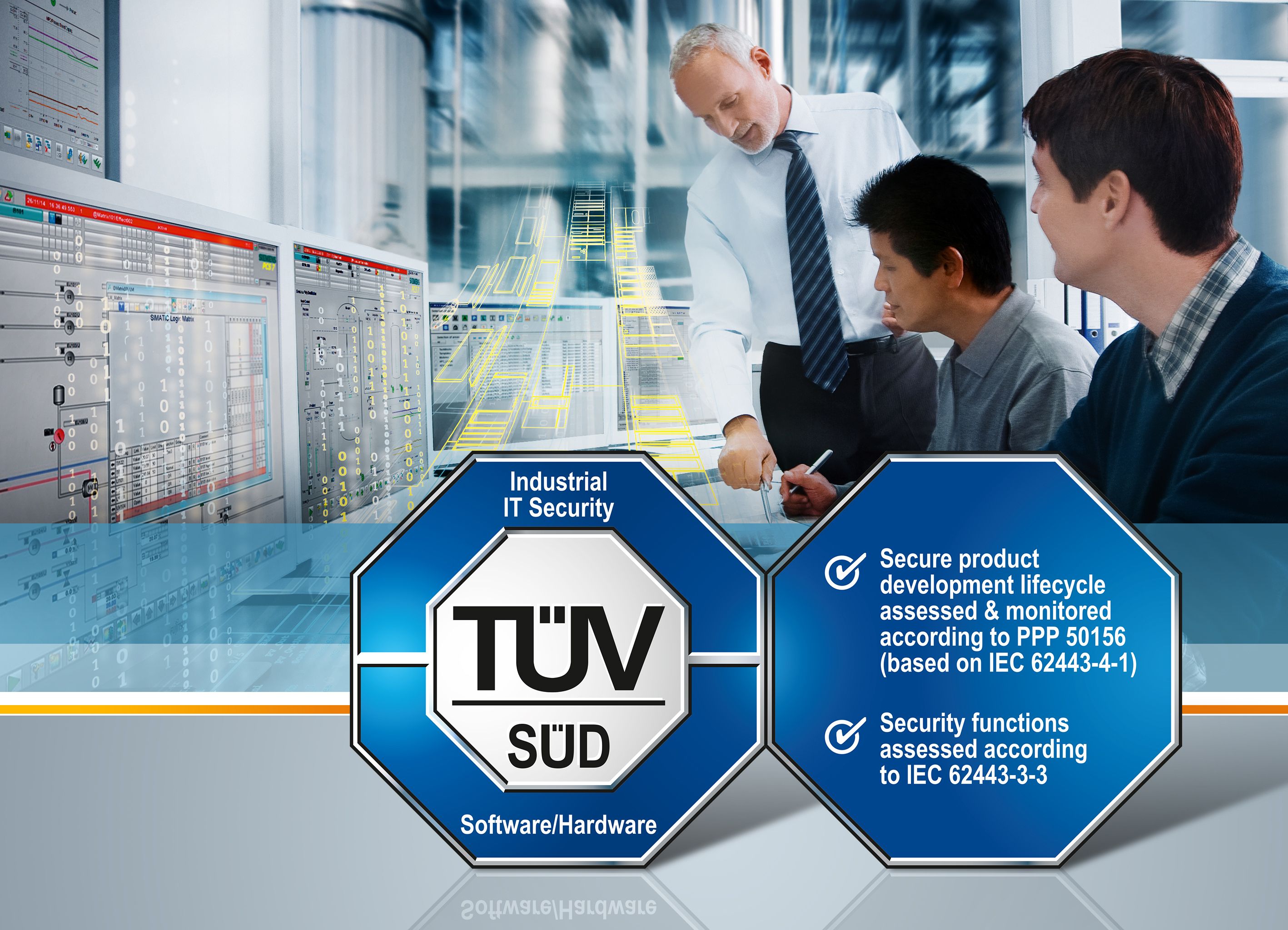
Siemens process control system first product with IEC 62443 security certification | Press | Company | Siemens

PDF) Central Institutional Ethics Committee needed to facilitate timely review of multicenter clinical trials